- #StopSwappingAir - February 20, 2021
- Why I fled - September 15, 2020
- 5 years later: Ebola outbreak shows continued weaknesses of global response - June 23, 2019
Please cite this article as: E.A. Kelser, Melioidosis: A Greater Threat Than Previously Suspected?, Microbes and Infection (2016), doi: 10.1016/j.micinf.2016.07.001.
Abstract
Research released in 2016 shows that the global burden of Burkholderia pseudomallei infection is probably much higher than previously suspected. Better testing and reporting are needed if we are to detect outbreaks and diminish deadly delays in treatment. Worldwide, researchers need support for vaccine development, particularly for public health vaccines to protect people and animals in endemic areas. Because melioidosis can infect so many species, collaboration and communication between human and veterinary medicine experts will be key to tracking, treating and preventing B. pseudomallei infections.
Melioidosis: A Greater Threat Than Previously Suspected?
Infection with Burkholderia pseudomallei may be more common and deadly than we think. Melioidosis is often thought of as a tropical disease affecting people and animals in Thailand, Northern Australia and SE Asia [1], [2] and [3] but a January 2016 article in Nature Microbiology[1] provides evidence that melioidosis may have a much greater endemic area, morbidity and mortality than previously suspected. With no vaccine available, possibly 89,000 human deaths (95% credible interval 36,000 – 227,000) and countless animal deaths per year, plus almost half the suspected endemic countries not reporting the disease, education about melioidosis is urgently needed among human and veterinary clinicians, laboratory staff, and policymakers worldwide.
In 1911, the pathologist Alfred Whitmore and his assistant C. S. Krishnaswami first described melioidosis as a “glanders-like” disease in Burma. During the last century, the etiologic Gram-negative bacillus has been variously known as Bacillus whitmorii (or Bacille de Whitmore), Malleomyces pseudomallei, Pseudomonas pseudomallei, and, since 1992, Burkholderia pseudomallei [4].
About
Melioidosis (aka “Whitmore disease” [5] and [6] “Whitmore’s disease” [3]and “pseudoglanders” [5] comprises a mixed purulent and granulomatous response that can lead to suppurative or caseous lesions of the skin or any other organ [5], [6] and [7]. In humans, the illness often presents as pneumonia [6] and [7] but also as sepsis or internal organ abscesses, [1], [2], [3], [6], [7] and [8] and suppurative parotiditis in children [4] and [6]. There is high case-fatality in both animals [4], [5] and [6] and humans [1], [2], [4], [6] and [7], up to 40% in resource-poor environments [7]. Chronic disease can also occur and mimic conditions such as tuberculosis or cancer [2] and [9]. Melioidosis has been traditionally difficult to diagnose because of its diverse clinical presentations and lack of adequate diagnostic capabilities [2] and [6]. Melioidosis is sometimes called “The Great Imitator” [6] or “Great Mimicker” [9].
Although the incubation is generally 1-21 days in humans [3] and [8] melioidosis has been known to present up to six decades later [2], [6] and [8] when activated by a weakening of the immune system [6], [8] and [10]. This potentially long incubation necessitates a complete travel history to see if there has been any exposure to endemic regions, or to plants and animals from those regions [2]. In light of recent evidence, that region may be much larger than previously suspected [1].
Numerous wild and captive animal species are susceptible to infection, with infection most often seen in sheep, goats and pigs. Introduction of naïve livestock to endemic regions appears to increase risk, as do immunocompromising conditions in dogs and cats. Diverse species affected include camels, wallabies and koala, tropical fish, captive marine mammals, tropical fish, birds, reptiles and non-human primates. Host susceptibility and disease manifestations vary between species [5] and [6]. Pigs seem to be more resistant to systemic infection than sheep and goats, and infections in cattle are rare [6]. Mastitis is a common manifestation in goats, leading to recommendations for pasteurization of goat’s milk in endemic regions [5] and [6].
Transmission
Transmission of B. pseudomallei is generally environmental [5] and [6]. The bacillus can survive for months or years in contaminated soil or water, including acidic soils, dehydrated soils, and soils with salt contents up to 0.4%. It does not survive UV light [4] and [6]. B. pseudomallei can exist in an infectious, non-cultivable state in the environment for prolonged periods. It can change its morphology to appear as Gram-positive coccoid organisms in acid pH, and also enter the cells of some protozoa or the mycorrhizal fungus Gigaspora decipiens, which may help B. pseudomallei to survive environmental stresses [11].
The bacillus often enters the system percutaneously, such as through open wounds on the hands or feet [2], [3], [5], [6] and [8]. It is often found in soil [3], [5] and [7], particularly moist clay “acrisol” soils [1] and [6] and heavily-worked “anthrosol” soils [1] such as are found in rice paddies [4] and [7]. It can also be found in surface water [2], [4], [5] and [6], and infection can occur from ingestion of contaminated water [3] or inhalation of contaminated dust and water droplets [3] and [5], including during severe weather events [4], [6] and [12]. There have been cases of infection from ingestion of or percutaneous exposure to contaminated milk [5], [6] and [10] and ingestion of or percutaneous exposure to contaminated carcasses [5] and [10]. Transmission does not usually occur from person to person or animal to animal, except transplacentally [5] and [6] or in families with close contact, such as nursing an infected patient [6].
Diagnosis
For melioidosis diagnosis, the recommendation is to suspect infection with B. pseudomallei in patients who present with community-acquired sepsis, pneumonia or abscesses, who have lived in or traveled to areas where the bacteria is endemic, particularly in the presence of predisposing risk factors such as diabetes, kidney disease, immunosuppression [2], [6], [8] and [9], excessive alcohol intake [1], [6], [8] and [9], lung disease [6], [8] and [9] or thalassemia [6]. Delays in diagnosis and appropriate treatment can often be fatal [1], [2], [4], [6] and [13]. In some symptomatic and asymptomatic patients, abscesses are found post-mortem [6].
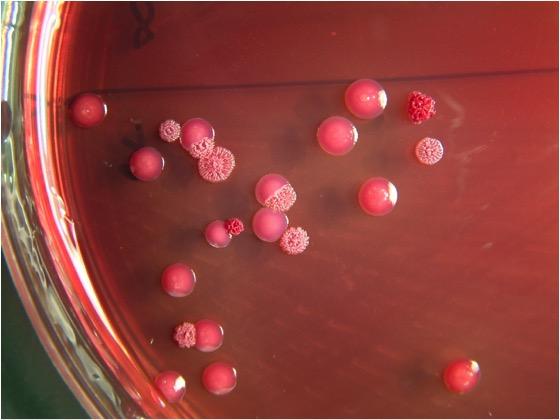
Sampling from multiple sites is more likely to detect the organism [6]. Sample urine, pharynx, blood and pus, on selective media (Ashdown agar, if available, but B. cepacia selective agar may be more commonly available) and transport at room temperature, as the bacillus does not survive cold temperatures well. Culture is 100% specific, but sensitivity may be as low as 60%. It is strongly recommended that any non-Pseudomonas aeruginosa, oxidase positive, gram-negative bacillus isolated from a clinical specimen be suspect [2]. It can be distinguished from closely-related B. thailendensis because B. pseudomallei cannot assimililate arabinose [7].
If melioidosis is suspected, the receiving lab should be notified, as risk for occupational exposure via aerosolization is high [9]. Additionally, because B. pseudomallei is a select agent, the lab may need to notify appropriate authorities (such as the Laboratory Response Network in the United States). Work with the sample should be performed in a BSL-3 lab [6] and [10]. If melioidosis is suspected, but not immediately found, it is recommended to search for occult foci of infection with imaging [2]. Genetic testing may also be needed to distinguish between various Burkholderia spp. [13].
An IHA titer >1:160 is sometimes considered diagnostic for melioidosis in resource-poor settings [7]. ELISAs have also been developed for the exotoxin and bacterial components, as well as PCR testing, PFGE, 16S rRNA sequencing, variable number tandem repeat polymorphism and multilocus sequence typing (MLST) [6]. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) is also increasingly used for isolate identification [2] and [6].
There is support among researchers to make latex agglutination testing available for rapid identification, especially in resource-poor endemic areas. Additionally, a point-of-care lateral flow immunoassay (LFI) could help to rapidly identify isolates [2]. In non-endemic areas, broader detection tests such as 16S sequencing or multiplexed real-time PCR assays that include B. pseudomallei may be helpful to catch occasional cases or potential outbreaks [2].
Misconceptions and Misidentification
Although chronic infection has been described, B. pseudomallei does not generally colonize people. Isolation of B. pseudomallei from any body site should be considered diagnostic of infection. B. pseudomallei is often misidentified as a contaminant or as Pseudomonas spp [2] or other Burkholderia spp [2], [4] and [13] by standard identification methods, including API 20NE [2] MALDI-TOF and VITEK2 [2] and [10].
Treatment
Bacteremia can recur after days or weeks of seemingly adequate IV treatment [4], prompting the treatment guidelines that last for months. For the first 10-14 days of treatment, IV Ceftazidime is given q 6-8hours OR IV Meropenem q 8hours, followed by 3-6 months of PO TMP-SMX q12 hours [3] and [14]. Recommendations are sometimes extended for certain clinical presentations including lymphadenopathy, ICU admission, osteomyelitis, central nervous system infections and arterial infections [14]. Livestock are often destroyed rather than treated [5] and [6].
Vaccines for Military and Non-Military Use
There was a high seroprevalence (7%) of B. pseudomallei among soldiers returning from Vietnam [4], and much funding for vaccine development is for military use to prevent infection of soldiers in tropical areas or in the event of intentional release, as there is the possibility for use as a bioterrorism weapon [4] and [15]. Although the potential exists, the likelihood of B. pseudomallei being used for an “attack” is believed to be low. It has never been known to be weaponized, and its use against an immunocompetent population is undetermined [4] and [16].
The need is greatest for a public health vaccine helpful for those most affected—percutaneous exposures to immunocompromised patients in endemic areas, not inhaled exposures to young, healthy soldiers. For this reason, there is concern that a military vaccine would have limited usefulness as a public health vaccine in endemic areas [15].
Attempts to vaccinate laboratory animals has led to abscess formation [4] and [15] even with species believed to have lower virulence factors such as B. thailandensis. Additionally, repeated environmental exposure to B. pseudomallei does not appear to elicit protective humoral or cellular immunity [4]. For vaccine development, an external agency that could facilitate head-to-head comparison of vaccine candidates may be needed [10] and [15]. Even with such an agency, vaccine development is an estimated 5-20 years away [10].
Reporting in The United States
Mainly, melioidosis is reported to authorities in SE Asia, South Asia, China, Singapore, Taiwan and Northern Australia, but indigenous cases have also been documented in the Middle East, South and Central America, Africa and various islands [6]. Reporting of melioidosis in areas where it is less common could help to educate clinicians, laboratory personnel and public health officials to be alert to potential cases and outbreaks [2].
In the United States, melioidosis is not nationally notifiable, despite being a Tier 1 overlap select agent and Category B Priority Pathogen. Melioidosis is the only overlap select agent (bioweapon potential against humans and animals) where this is the case. Although identification of B. pseudomallei or occupational exposures must be reported to the Federal Select Agent Program within 24 hours, it is not required for states to report suspect or probable cases of human infection to the CDC’s Bacterial Special Pathogens Branch [9].
There have been U.S. cases where there was no foreign travel and the source of exposure was unknown [2], [6] and [9]. The authors of the Nature Microbiology paper propose that the U.S. states of Florida, Texas and Louisiana have soil suitable for B. pseudomallei to become established. [1] But the reporting websites of those 3 states show a patchwork of reporting by the counties to the state—urgently notifiable in Florida, reportable within 5 days in Louisiana, not reportable at all in Texas.
In the U.S., national notifiability is determined by the Council of State and Territorial Epidemiologists (CSTE), who have declined to make melioidosis nationally notifiable. As far as we know, the disease is rare in the United States, and some of the blood testing to identify B. pseudomallei is already performed at the CDC [17]. For veterinary patients, B. pseudomallei is currently on the USDA’s proposed National List of Reportable Animal Diseases [18].
How might prevalence increase?
The vast increase of areas where melioidosis may be endemic necessitates increased awareness among human and veterinary clinicians, lab personnel, and public health officials. We can expect to see an increase in reported cases if diagnosis and reporting improve, but other factors may also drive an increase in cases in coming years—worldwide increase in diabetes, increase of anthrasol soils, and increasingly global movement of people, animals and goods. [1].
Recommendations
Better testing and reporting are needed if we are to fully understand the global burden of melioidosis. We also need faster testing and increased awareness of the disease in order to prevent deadly delays in treatment.
In the United States, reporting may be indicated to see whether the numbers of imported and endemic cases begin to rise. Without such reporting, it will be difficult to detect outbreaks or the possible intentional release of the organism.
Worldwide, researchers need support for vaccine development, particularly for public health vaccines to protect people and animals in endemic areas. Because melioidosis can infect so many species, collaboration and communication between human and veterinary medicine experts will be key to preventing, tracking and treating B. pseudomallei infections.
1. Biosketch of T. Eoin West
Dr. Eoin West is a pulmonologist/intensivist at Harborview Medical Center, Seattle, WA, USA, and an Associate Professor of Medicine in the Division of Pulmonary and Critical Care Medicine and adjunct Associate Professor of Global Health at the University of Washington. He is a Visiting Associate Professor of Clinical Microbiology in the Faculty of Tropical Medicine at Mahidol University. He conducts translational research focused on lung infections and sepsis, illnesses that cause a significant burden of disease in low resources settings. Much of his work investigates melioidosis, Burkholderia pseudomallei infection. He directs the UW’s International Respiratory and Severe Illness Center (INTERSECT). Figure 4.
2. Interview with T. Eoin West
-
Erin Kelser (E.K.): You saw the article by Direk Limmathurotsakul in the January 2016 issue of Nature Microbiology. Do you also suspect that the prevalence of melioidosis may be higher than previously supposed? T. Eoin West (T.E.W.): I do. The diagnosis of melioidosis is really quite dependent on adequate diagnostic facilities and capabilities, and in many tropical regions where the bacterium could live in the environment, the diagnostic microbiology capacity is inadequate. So I do think that there is likely to be more melioidosis than we are currently identifying. In a way, the more one has looked for it in regions where it’s been suspected, the more it’s been found. E.K.: Your specialties are pulmonology and critical care medicine. How did you get interested in melioidosis? T.E.W.: I am a pulmonologist, an intensivist, and also a researcher. I’m mostly interested in lung infections and sepsis, sepsis being the dysregulated host response to infection resulting in organ failure. I started roughly 10 or more years ago working on melioidosis because it’s a disease that presents in many different ways, but involves the lungs in 50% of the cases. It’s a disease that results in sepsis – Gram-negative sepsis, specifically. It’s a disease that’s of interest I think to people working on infectious diseases, and lung doctors, and intensive care personnel because the disease is so severe in parts of the world. Northeast Thailand and Cambodia are two examples of somewhat lower resource settings where the mortality rate is at least 40%. The mortality rate is lower in Northern Australia, and in places like Singapore where there are more and better ICU facilities.It’s a fascinating disease and the organism is quite remarkable. It’s an active public health threat, not only in Southeast Asia and Northern Australia, but for many of us in the melioidosis community there’s been a growing concern that it’s a problem elsewhere and has yet to be diagnosed. An example is South Asia. In fact, this past year there was the first melioidosis congress in South Asia. I didn’t go to it, but I think that conference was representative of the concern amongst Indian clinicians that they were seeing more and more melioidosis and needed to tackle it.From my perspective, it’s a lung disease, it’s an infectious lung disease, it causes sepsis, and it makes patients critically ill. It has a high fatality rate in many places, and I should point out that the high fatality rate in places like Thailand are generally despite appropriate antibiotic therapy. Maybe the ICU care is a little bit different in Northeast Thailand compared to say in Northern Australia where the case fatality rate is lower, maybe the bugs are slightly different, maybe the hosts are a little bit different, but even though many patients in Northeast Thailand get the right antibiotic up front, it’s still very lethal and hard to treat. It takes months and months of therapy to really eradicate the infection. It’s a big problem that I think this modeling paper suggests is larger than previously recognized, but I think the conclusions in this paper are ones that most of the melioidosis community would have not found surprising. So there’s a lot of work to be done. E.K.: Are there things that you find interesting about the organism Burkholderia pseudomallei itself? T.E.W.: I’ll preface this by saying that I’m a clinician and my main research interests are really in the host response. I’m not a bacteriologist. That being said, I think the organism itself is interesting because it’s got a big genome, and because it is intrinsically resistant to many different antibiotics. There’s also evidence that some antibiotics, to which the organism seems to be susceptible in vitro, do not work very well clinically, further limiting the choices. I think that makes it interesting. Moreover, the organism is a facultative intracellular pathogen, so it can survive in various different cell types and it can move from cell to cell. It’s also interesting because the disease presents in many different ways. You can get infected by inoculation through the skin. You can get infected by inhaling the organism. You can get infected by ingesting it. There are numerous routes of infection, and honestly it’s often tricky to tell for specific cases how the inoculum occurred. For example, many of the cases in Northeast Thailand are agricultural workers, rice farmers who are out in the fields. They’re often barefoot and may have cuts and scrapes. They’re in rice paddies where the organism exists, and they’re also exposed to winds and rain. Also, they’re drinking local water. So it’s not necessarily clear– are they getting infected because it’s getting through their skin when they’re out working, or is it because they’re inhaling it during heavy rains in the rainy season causing aerosolization of the pathogen, or are they actually getting it because they’re ingesting it in their water supply? In terms of the manifestations of disease there’s tremendous variability. The organism causes pneumonia in roughly 50% of the cases, but it can also cause a more localized skin infection. It can infect the joints, it can infect the neurological system, it can cause abscesses in the spleen, in the liver, in the prostate. In kids in Thailand you can see parotitis, which is inflammation/infection of the parotid gland. You can of course get bloodstream infections. There’s tremendous variability in what the disease will look like when patients seek care. Then the other thing that’s interesting is the variability in duration from exposure to development of symptoms of infection. Some cases have been reported with a very long duration of time – many years – between the exposure to the contaminated environment and actual manifestation of disease. Honestly, those aren’t terribly common. Most patients seem to present with disease sooner, although one of the challenges is that most people who present with disease are people who are continuously exposed to a contaminated environment, so one of the challenges is knowing when exactly they got infected. In Northeast Thailand where there are a lot of data on this disease, we know that the majority of people have antibodies to Burkholderia pseudomallei by their teenage years. People are exposed to it, but only a minority of people ultimately develop disease. It’s possible that there’s a form of latent infection that’s recrudescing at some later stage. We also know that the people who get infection tend to be abnormal hosts, meaning they don’t have a normal immune system. They’re often diabetic or they may have a history of heavy alcohol use or chronic kidney disease for example, some major risk factors that predispose people to infection perhaps. But again, with respect to what makes the organism and disease fascinating is really this variability in the timing of exposure and manifestation of disease. Then the other thing is the challenge in eradicating it. Most bacterial infections you can get rid of with a course of antibiotics lasting one to two weeks at most. This bacterium is really much, much, harder to eradicate. You need weeks, initially several weeks, of intravenous therapy followed by oral therapy for months, and even then there’s still a concern about developing relapse where the organism has still not been completely cleared. That raises the question “Well, where is the bacterium hiding out?” It makes me think a little bit about a disease like tuberculosis. For tuberculosis, there’s a latent form of infection and then people tend to develop active disease at a later stage, and it’s really hard to get rid of. You need months of therapy. There are some parallels between melioidosis and tuberculosis. E.K.: Does Burkholderia pseudomallei have ways of getting around host responses? T.E.W.: Well, its interaction with the immune system is complex, and I don’t think we fully understand this by any means. It does manage to live intracellularly and move from cell to cell, so in that sense yes, it is getting around the host defenses. It escapes from the phagosome of the cell that has engulfed it, and then it gets into the cytoplasm and can run amok. It does activate the immune system in other ways too, but I think the immune system in many cases fails to control it.I think it is important that most people who get melioidosis are not totally healthy. They’ve got some abnormality of their immune system – diabetes, heavy alcohol use, chronic kidney disease, things like that. The bacterium is definitely able to take advantage of people in that state. The bottom line there is we don’t fully understand the interaction of the organism with the host at all. I spend a lot of time thinking about the interaction of the organism with the host and there’s a lot that we don’t know. There are some other organisms like Francisella tularensis which I would say definitely evade any recognition by the immune system very, very effectively and Burkholderia pseudomallei is different from those. There have been some publications previously suggesting that the endotoxin or the lipopolysaccharide (LPS) for B. pseudomallei is somehow less inflammatory and it produced less of a host response to the bug when infecting people, but actually in work in my lab and with collaborators from Mahidol University we’ve not found that really to be the case. We think that B. pseudomallei is pretty inflammatory, and our tests don’t support what was previously published. E.K.: As a clinician, when would you recommend people think Burkholderia in the case of either being in an endemic area or in a non-endemic area? T.E.W.: Well, in an endemic area you have to think about it all the time, and there are some examples of places where that’s really happening. For example, in Northeast Thailand where there’s been a lot of work on the infection in years past – really seminal work – and similarly in Northern Australia, clinicians are attuned to this possibility and have a low threshold to start the appropriate antibiotics. They recognize people coming in sick with something that looks like pneumonia or abscesses or fever and sepsis, particularly patients with risk factors for getting infected. They are in many cases thinking about this infection and starting the appropriate antibiotics well before there’s any microbiological diagnostic information, which is really the best way to manage folks, because it’s going to take a couple of days for your cultures to come back or turn positive. You don’t want to be treating people incorrectly during that time frame.Now, there’s a lot of work to be done in other endemic areas where clinicians aren’t so familiar with melioidosis and I’ll give you an example: Cambodia. Melioidosis is well described now in Cambodia. There’ve been a number of papers over the last few years that have reported it, but it’s a disease that is not familiar to most clinicians and I think there are probably a lot of cases of melioidosis that go completely unidentified and untreated. So there’s an endemic area where lack of familiarity on the part of the clinicians means that there’s probably inadequate diagnosis or treatment. The problem is compounded by the fact that in countries like Cambodia, the diagnostic microbiological facilities may not be sufficiently advanced to detect the organism and then in some cases too, the appropriate antibiotics aren’t available. India, I think could be another country where it’s clear that melioidosis is there, but it would be a disease that for many clinicians it is not something that they’ve been trained about. The challenges are many fold: to educate people about the disease, it’s important to ensure that the microbiology facilities are capable of identifying the organism, and it’s important to have the appropriate antibiotics available. They’re not always available so then even if you have the other pieces of the puzzle in place but you can’t treat somebody then it is all moot. What you’re looking for as a clinician is for people who have risk factors, and you’re looking for people who are presenting basically with pneumonia or sepsis. The challenge with this disease is that it can present in many different ways, but if you’re thinking about infection in an endemic area one of the things you have to consider is B. pseudomallei. For clinicians in non-endemic areas, then most cases – for example in the United States – are imported cases. And so a good travel history is critically important as is understanding what the potential pathogens are, to which people might be exposed. Southeast Asia is a place where melioidosis is on the map. But I’m not sure that people would think about melioidosis at the top of the list in individuals who travel to say Brazil or India, other places where melioidosis exists. There’s also recent evidence of melioid in West Africa in Gabon. I think that the challenge here for clinicians in managing patients who’ve traveled is that the zone of endemicity is probably larger than many people know. So even if clinicians have heard about melioidosis, they may not be thinking about it in the various countries that are being visited by their patients. Then the other consideration is an intentional release scenario. Burkholderia pseudomallei is a bio threat agent, so then the concern would be in the right scenario, or if there were a cluster of cases geographically located, could there have been some intentional release? To my knowledge it’s never been used as a biological weapon, but it is as you know a select agent, a Tier One select agent, so it’s viewed as potentially exploitable for nefarious purposes by the U.S. Government. E.K.: My background is in infection control and with communicable disease investigation for a county. I’m concerned that B. pseudomallei is a Tier One select agent, but it isn’t nationally notifiable and it isn’t reportable in most states. I’m concerned that an outbreak or intentional release may not be caught immediately because it wasn’t reported to public health. Is that cause for concern? T.E.W.: Well it’s interesting. Melioidosis in the United States is almost always imported. I think that you raise a good question. In the event of a bio threat scenario, an intentional release, how would that be identified if there isn’t a requirement to report it? Because I think that’s the main thing one would worry about. It’s also conceivable that an outbreak could occur if the environment was contaminated. For example did you read about L’affaire du Jardin des Plantes? You know the index case was supposed to be the panda that Mao Tse Tung had given as gift to Georges Pompidou, and then a whole host of other animals died in the Paris zZoo and the Jardin des Plantes menagerie. I looked into this last year, and they the deaths weren’t all in the immediate proximity of each other. There was the Jardin and then the Paris Zoo, and they’re actually slightly separate locations in Paris. They both have animals I believe. The outbreak spread elsewhere in France, too. The environment had somehow been contaminated. Perhaps Tthe infected panda was infected and infected bedding, and the bedding may have been moved around, or people wore dirty boots and they moved the bacteria around into other enclosures and to other sites, and even the though the Zoo was not quite adjacent to the Jardin, there was an outbreak. I suppose one could envision something like that happening, where there’s some contaminated materials that wereare brought over and essentially seed the environment, or a contaminated animal which then spreads itbacteria around via feces somehow. I suppose that’s all feasible, so either that sort of a scenario or an intentional release scenario, and if it’s not a reportable disease then I think you’re right, that would suggest there would be a lag in identifying it. From my perspective as a researcher, we’re interacting mostly with the CDC and all of our activities with Burkholderia pseudomallei are governed by the U.S. regulations and overseen by the CDC. My understanding was that even if it’s not notifiable to local public health authorities, you may have some obligation to report a clinically isolated select agent if you’re going to keep it. My understanding was that once you had some Burkholderia pseudomallei popping up in a clinical lab, you basically had to declare it as a Select Agent or destroy it within seven days. E.K.: In the melioidosis community, is there a lot of collaboration with veterinary medicine, such as is found in the One Health model? T.E.W.: That’s a great question. I would have to say that there’s probably not as much that I’m aware of as there might be. Now I say that from my perspective, which is not of course all encompassing, but it is true thatand most of my interactions are with other clinicians and researchers who are not necessarily veterinarians. There may be an opportunity there for greater collaboration and you had mentioned the One Health notion, which tries to view consider environmental issues, veterinary and human diseases and bring them all together. I think that makes good sense. For example, in Australia there have been’s reports of melioidosis outbreaks in piggeries., and I know that there’s been a fair bit of work perhaps on the veterinary level because of the impact of the disease on pigs and I know that there are’s others in Thailand who studied the melioidosis in goats. Melioidosis can cause disease in all sorts of different creatures, mammals and others, but I’m not aware of a tremendous amount of medical veterinary information research underway from my perspective. That may just reflect my little human-focused angle, but when you brought it up the One Health model I thought, “Well yes, that’s an excellent notion.” I’m not sure it’s happening to the degree that maybe it could. E.K.: Is there anything else you can think of that is particularly important or pertinent for people to understand about melioidosis? T.E.W.: Well, I think what this modeling paper has shown (or is at least proposing) is that there is a lot more melioidosis in the world than we might recognize presently. I think tThat’s important because there’s tremendous potential impact if we can manage the disease better– if we can develop a vaccine, if we can reduce exposure to it, and if we can manage it more effectively. I think this paper will help raise awareness of the disease, not only in the areas where it’s currently an active public threat, but also to get people thinking about this broader zone of endemicity and the ramifications of the disease. In the United States, a lot of money is being put into select agent research in the last 15 years or so, with this concern of about biological weapons. While I understand that concern, what drives me forward as a clinician is really the active disease that exists is out there currently, the active public health impact of melioidosis. So I think that, while Burkholderia pseudomallei is a select agent and a potential bioweapon, there are thousands and thousands of people –, maybe up to 89,000 a year – dying of this disease. Take the model’s numbers that were published, that’s a really pretty profound impact, and there’s an awful lot of work to be done. There’s a lot we don’t know about the organism or really understand about the disease and the host defenses. This enhanced awareness, the attention that this article has received, I think it’s well justified. Of course the limitation is, that it’s just a model. and I’m not a modeling expert but it the model seems to have been carefully thought out. Direk Limmathurotsakul, the first author, is a collaborator of mine, and I know several of the other authors on the paper. They’re very extremely capable researchers, and many of them are clinicians. I definitely give them the benefit of the doubt, but it’s just a model. We don’t really know for sure the true burden, and I think that’s important to do. I mean, just in Cambodia, as an example where of a country where I’ve been engaged in some projects, the rolling out of diagnostic microbiology facilities, the basic capacity to do blood cultures or, to do sputum cultures for example have resulted in the identification of more cases of melioidosis. But that sort of capacity building is needed along with education of clinicians who maybe have managed for many, many years without microbiology facilities so, they don’t actually use them when they such testing become available. That sort of issue needs education too. T.E.W.: There’s a lot to be said–In short, there needs to be education about the disease, about how to diagnose it, about how to use diagnostic facilities. There needs to be capacity building in order to make the diagnosis, and then there needs to be the availability and proper use of the therapies. This is all in addition to any notion vaccine development and prevention efforts. There’s an awful lot to be done.
References
-
-
- Melioidosis Diagnostic Workshop, 2013
- Emerg Infect Dis, 21 (2015) http://dx.doi.org/10.3201/eid2102.141045
-
-
-
-
-
-
-
- T.J. Benoit, D.D. Blaney, J.E. Gee, M.G. Elrod, A.R. Hoffmaster, T.J. Doker, et al. Melioidosis Cases and Selected Reports of Occupational Exposures to Burkholderia pseudomallei—United States, 2008-2013. Morbidity and Mortality Weekly Report, 64 (ss05) (2015), pp. 1–9.
-
-
-
-
-
-
-
-
-
© 2016 Institut Pasteur. Published by Elsevier Masson SAS. All rights reserved.
Note to users:
Accepted manuscripts are Articles in Press that have been peer reviewed and accepted for publication by the Editorial Board of this publication. They have not yet been copy edited and/or formatted in the publication house style, and may not yet have the full ScienceDirect functionality, e.g., supplementary files may still need to be added, links to references may not resolve yet etc. The text could still change before final publication.